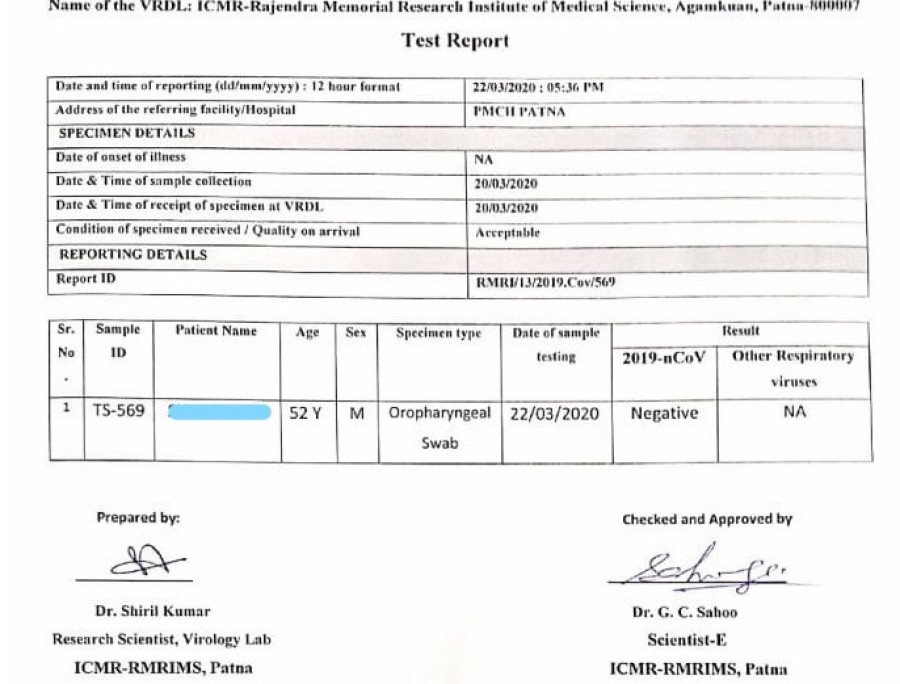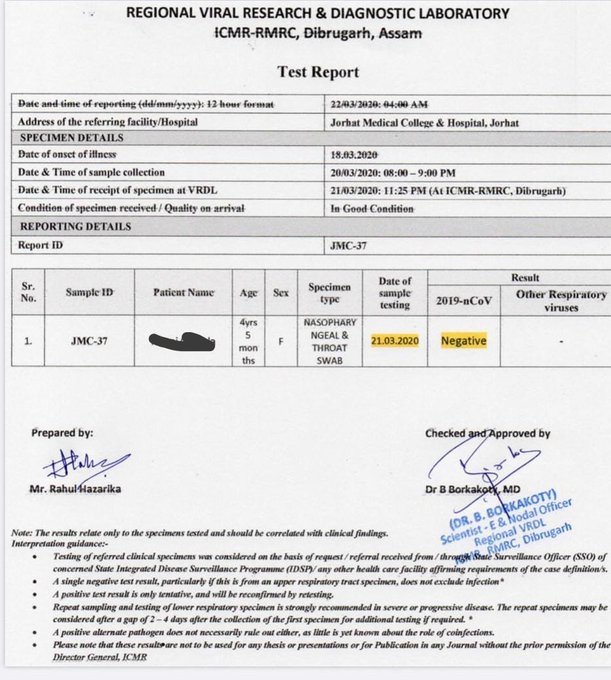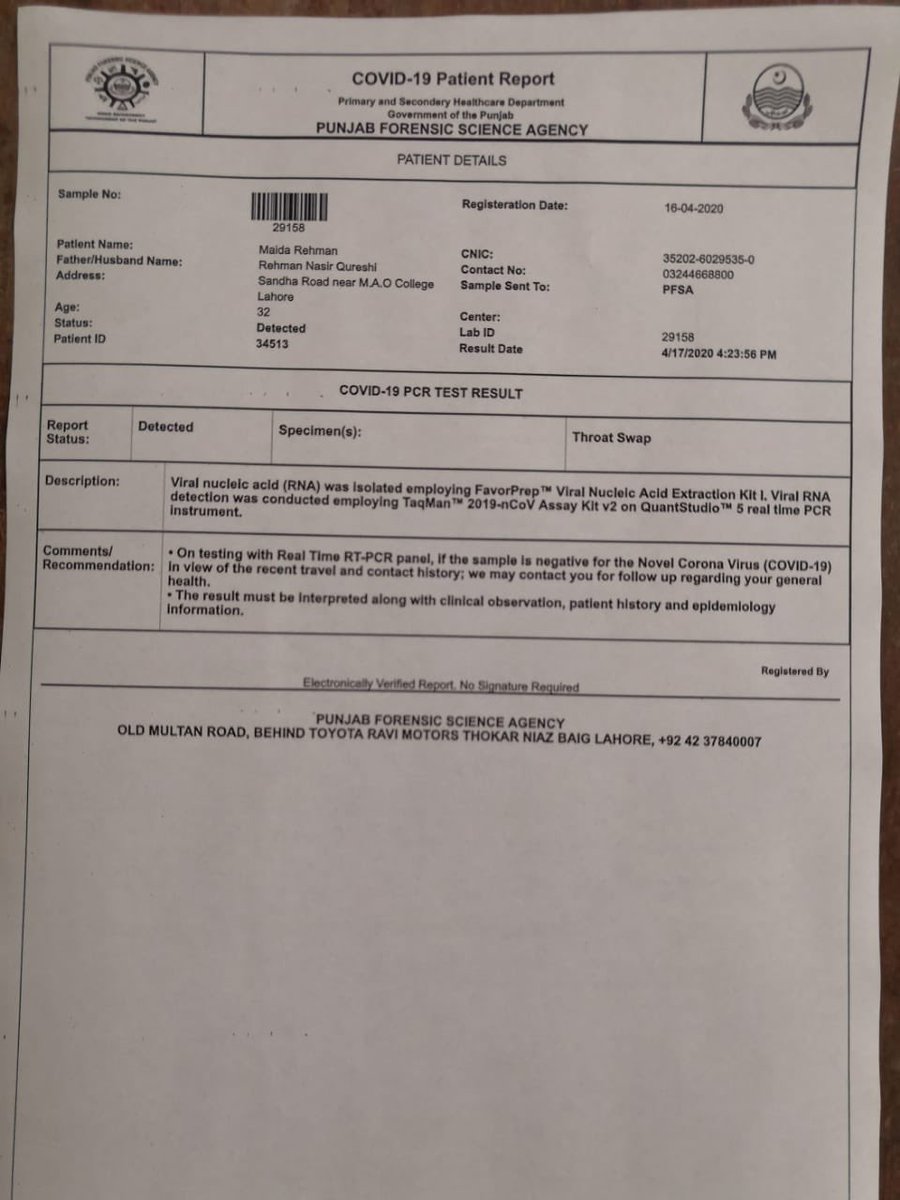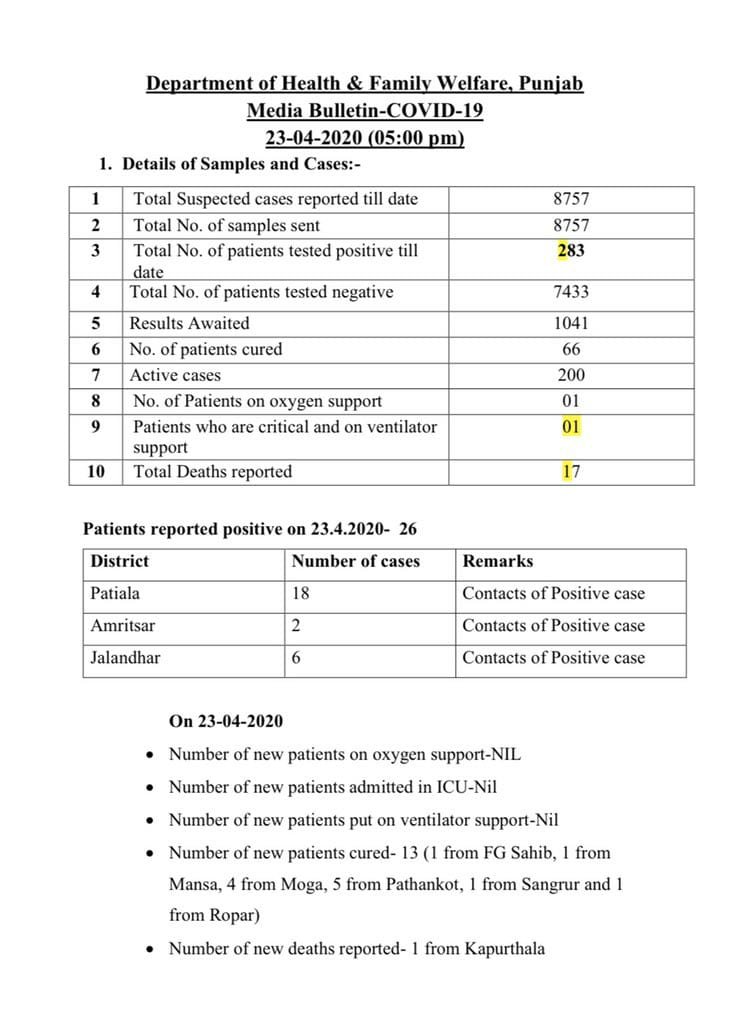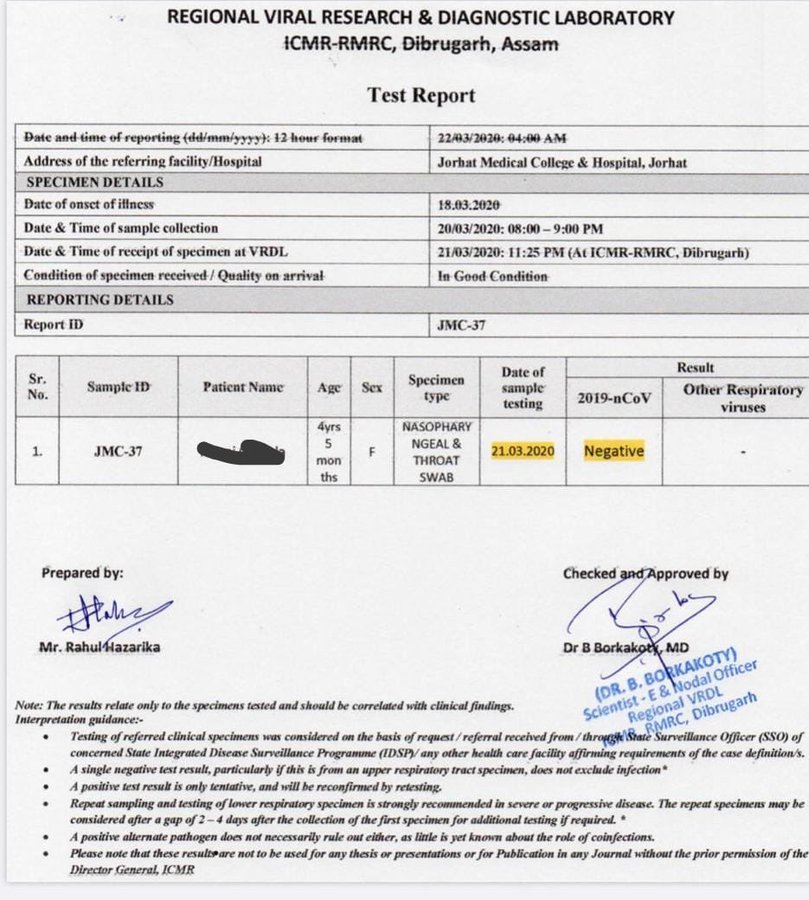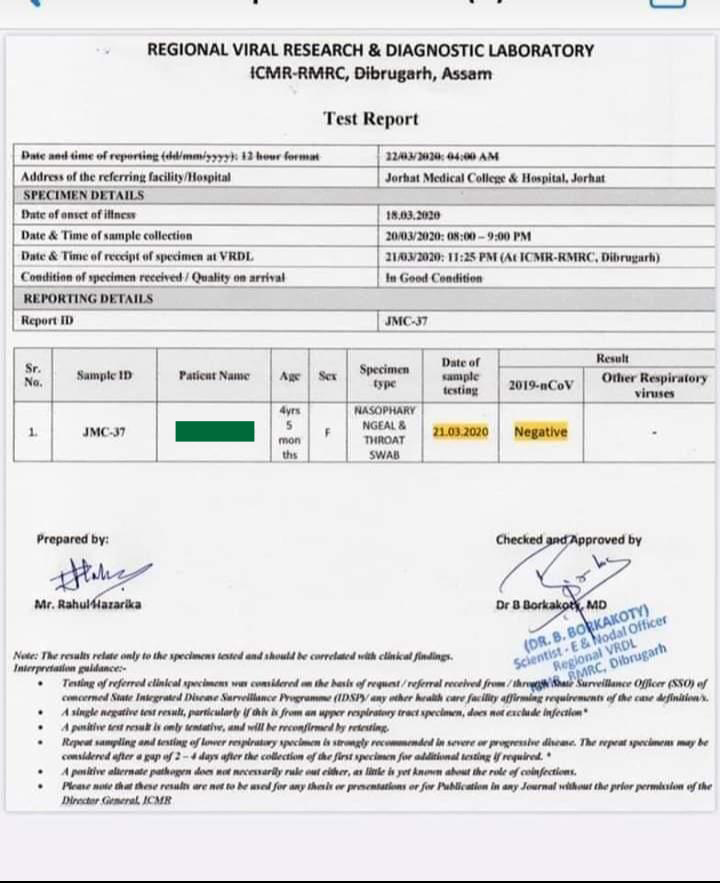
Either using a respiratory secretion sample from a nose and throat swab or a blood sample drawn from a vein. Coronavirus testing is primarily done with one of two kinds of samples. To prevent further spread of sars cov 2 and to collect information to better understand the virus and its impact on health outcomes cdc is working with state and local health departments to identify persons under investigation pui in the united states and test them for the virus that causes covid 19.

The fda is trying to tell us that this sars cov 2 test by bd gives some false positives. Next you could be one of the people who was not infected and the test gave you a false positive. A false negative result occurs if the sample s antigen level is positive but below the test s detection limit requiring confirmation with a nucleic acid test.

It can be deployed in laboratories or at point of care and gives results in 15 minutes. The test is simpler and cheaper but less accurate than nucleic acid tests. Psv practice game canceled due to positive opponent s corona test 2020 07 31t16 28 21 489z one of the opponents of the eindhoven team tested positive for the corona virus.

One report published june 23 in the journal jama network suggests that pooled testing for covid 19 could work as long as the positive test rate remains below 30 meaning fewer than 30 of tests. Florida labs not reporting negative test results report says fox 35 news ran an investigation of the figures contacting local locations listed in the state s report. When dna binds to specific probes a special type of light is produced that can be seen by the machine and the test shows a positive result for infection with sars cov 2 the virus that causes.