With our free coronavirus case report you can easily gather and organize these reports online so. A coronavirus case report allows people to report a suspected member of their workforce or community exhibiting covid 19 symptoms. Shared by stevengreenwood in coronavirus response forms.

Coronavirus case report template. A false negative result occurs if the sample s antigen level is positive but below the test s detection limit requiring confirmation with a nucleic acid test. It can be deployed in laboratories or at point of care and gives results in 15 minutes.

The test is simpler and cheaper but less accurate than nucleic acid tests. Monitor your symptoms and get medical help right away if you have trouble breathing confusion or bluish lips or face. A positive covid 19 test means you currently have or recently had the virus.

These are very fast and you might get an answer back in 15 minutes advantages. The antigen test is similar to what you would get with a rapid flu or rapid strep test mohler says. Covid 19 antigen tests detect viral protein in respiratory samples but these tests are not.

One type of covid 19 test detects the genetic material of the virus in a sample from the respiratory tract covid 19 serology blood tests detect antibodies produced in response to the infection. Covid 19 is the name of the infection and illness caused by the new strain of coronavirus called sars cov 2. The fda is trying to tell us that this sars cov 2 test by bd gives some false positives.

Next you could be one of the people who was not infected and the test gave you a false positive. Science s covid 19 reporting is supported by the pulitzer center. 22 2020 3 35 pm.
Quick and cheap but too often wrong.
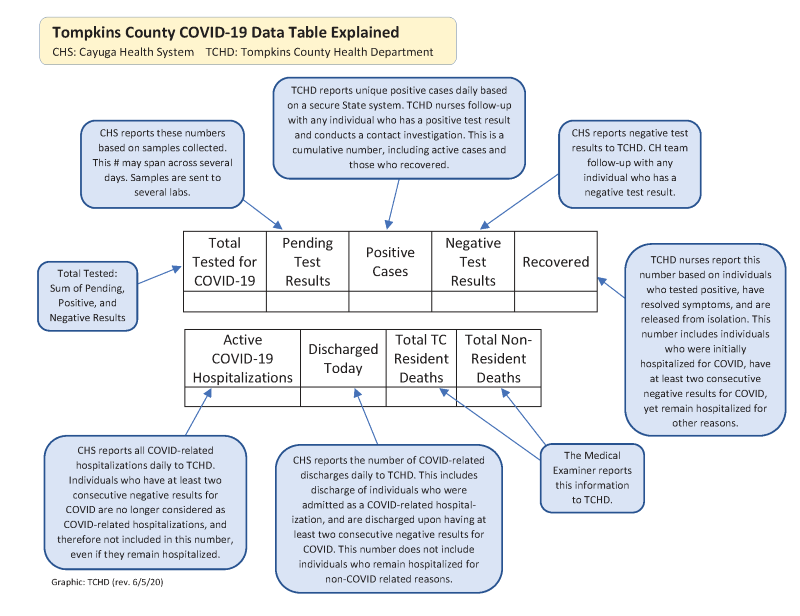
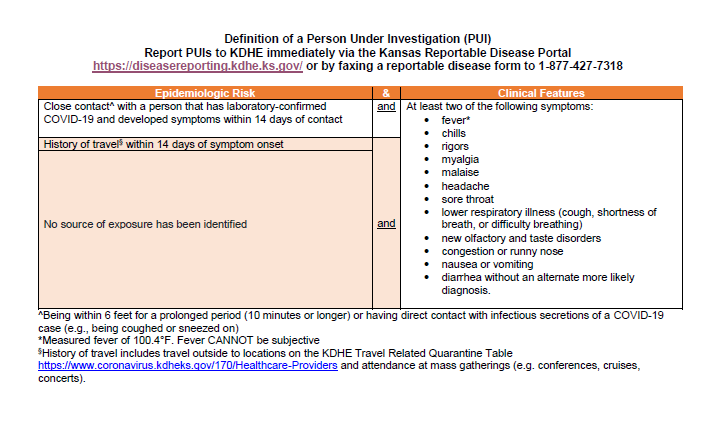
Corona test positive report format. The coronavirus aid relief and economic security cares act cares act section 18115 pdf icon external icon and its implementation guidance require every covid 19 testing site to report every diagnostic and screening test performed to detect sars cov 2 or to diagnose a possible case of covid 19 e g molecular antigen antibody to the appropriate state or local public health. To prevent further spread of sars cov 2 and to collect information to better understand the virus and its impact on health outcomes cdc is working with state and local health departments to identify persons under investigation pui in the united states and test them for the virus that causes covid 19. When dna binds to specific probes a special type of light is produced that can be seen by the machine and the test shows a positive result for infection with sars cov 2 the virus that causes.
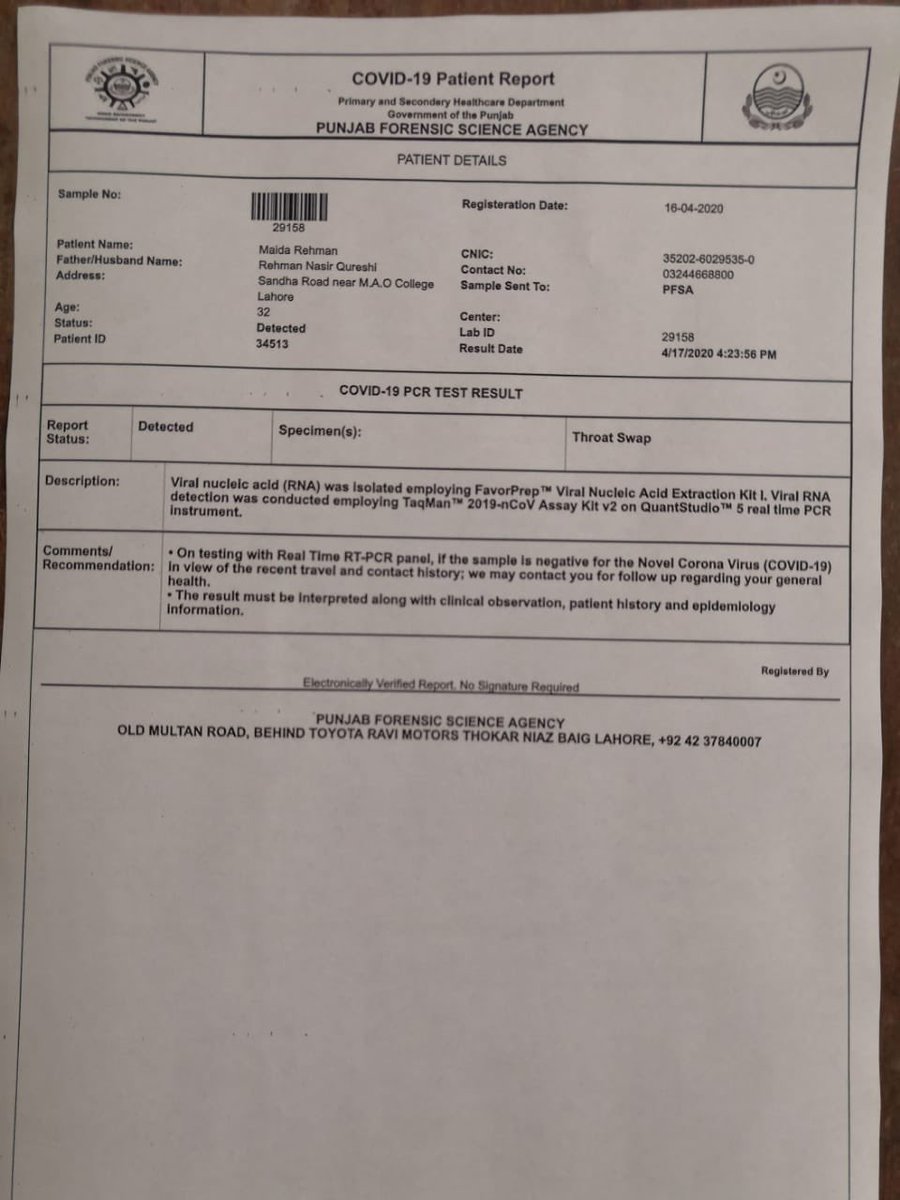
When dna binds to specific probes a special type of light is produced that can be seen by the machine and the test shows a positive result for infection with sars cov 2 the virus that causes. To prevent further spread of sars cov 2 and to collect information to better understand the virus and its impact on health outcomes cdc is working with state and local health departments to identify persons under investigation pui in the united states and test them for the virus that causes covid 19. The coronavirus aid relief and economic security cares act cares act section 18115 pdf icon external icon and its implementation guidance require every covid 19 testing site to report every diagnostic and screening test performed to detect sars cov 2 or to diagnose a possible case of covid 19 e g molecular antigen antibody to the appropriate state or local public health.