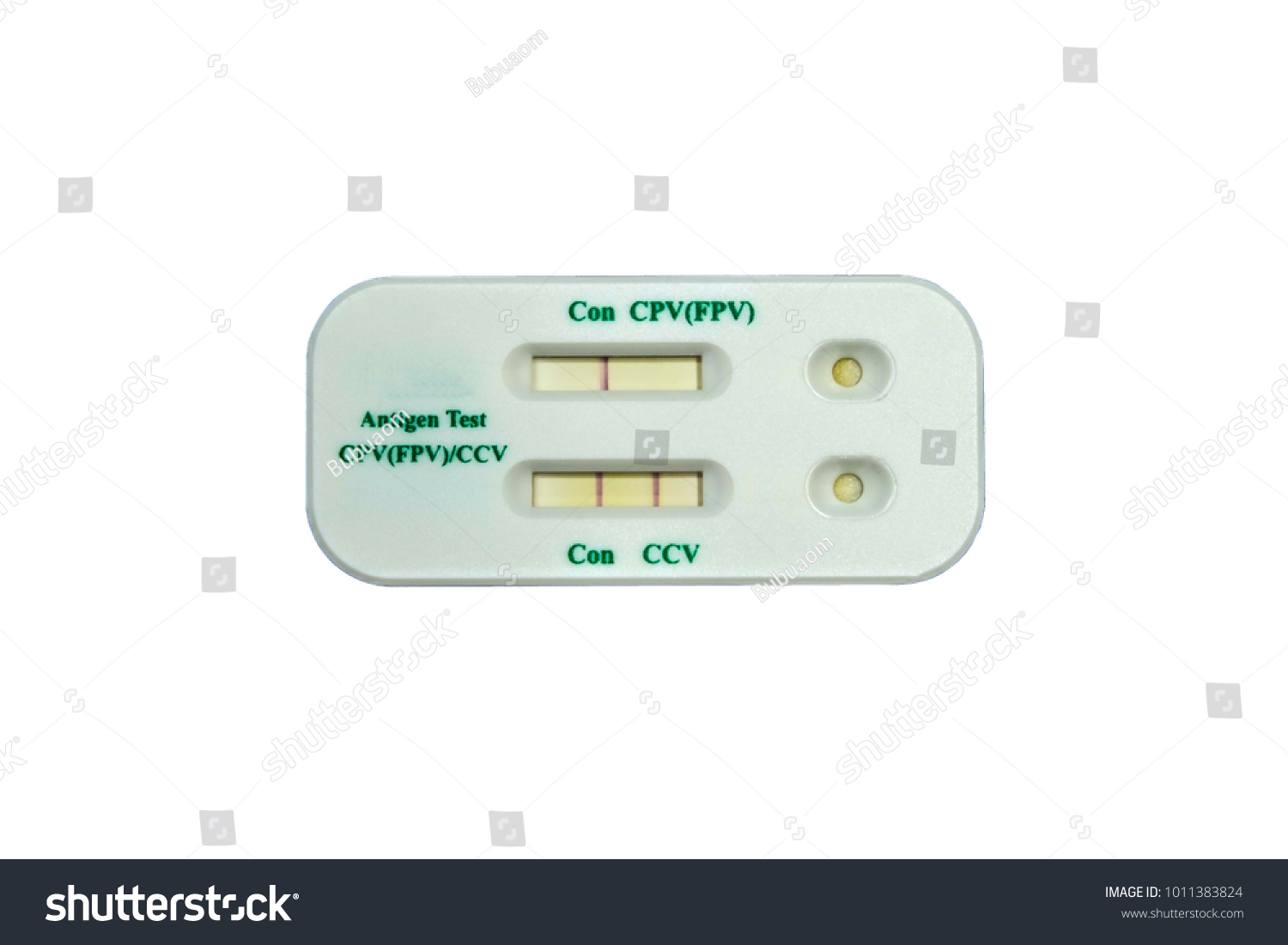
Tests for viral presence are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Covid 19 testing involves analyzing samples to assess the current or past presence of sars cov 2 the two main branches detect either the presence of the virus or of antibodies produced in response to infection. To diagnose a patient with this type of test kit the patient s nasopharyngeal swab sputum or alveolar lavage fluid would firstly be collected to get a sample of the virus.
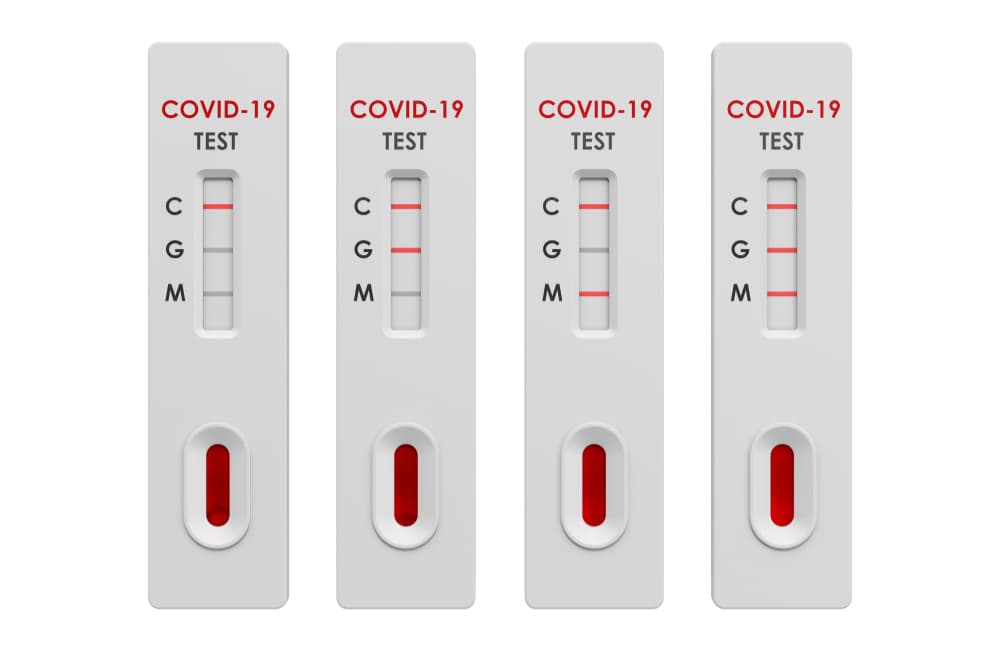
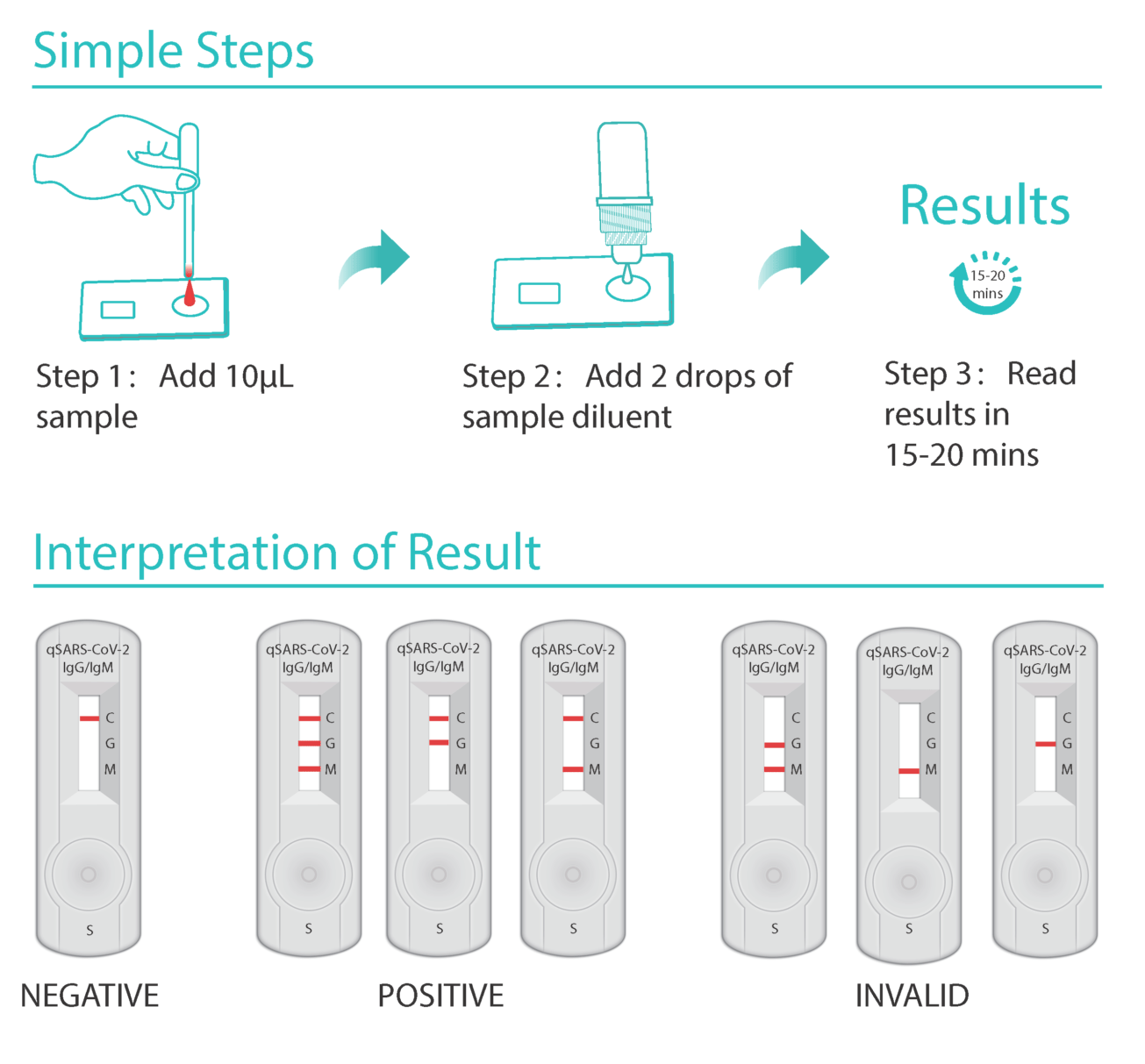
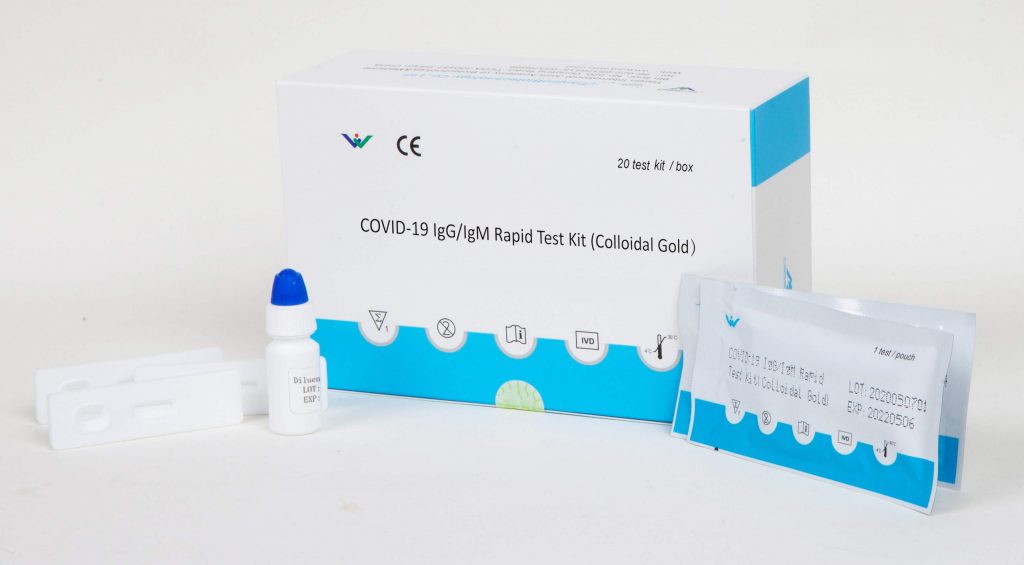
Since we ve already got the whole sequence of the ncov the test kits normally used this time at least in china fall into the nucleic acid class. It is a rapid test that identifies early and late combined igg igm antibodies of sars cov 2 the igg igm sars cov 2 rapid test kit provides you with results in 15 minutes and allows you to isolate carriers immediately. The cellex igg igm sars cov 2 rapid test kit is an accurate screening test for the diagnosis of covid 19 novel coronavirus.

Sars cov 2 igg igm rapid test. Coronavirus test kits used in tanzania were dismissed as faulty by president john magufuli on sunday because he said they had returned positive results on samples taken from a goat and a pawpaw. Covid 19 test kits in tanzania have raised suspicion after samples taken from a goat and a pawpaw fruit came back with positive results as the president said there were technical errors.
A federal review by the department of health and human services hhs found that the early version of the centers for disease control and prevention s cdc coronavirus test kits failed because. On the basis of clinical control trials held at nims university jaipur all the corona positive patients. The coronil anti covid tablet launched by baba ramdev on tuesday claims to recover corona positive patients within a span of 3 to 15 days.
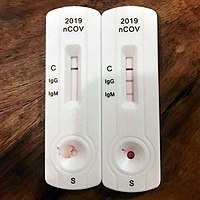
Patanjali s anti covid kit includes 3 medicines namely coronil tablet anu taila and swasari vati. The trump administration has reportedly ordered an investigation into a centers for disease control and prevention cdc lab in atlanta that was in charge of assembling coronavirus test kits after. The washington post reported on april 18 that the test kits had generated false positive results caused by the cdc s contamination at 24 of the first 26 public health labs that tried.
The study s lead author sin hang lee md director of milford molecular diagnostics laboratory found that the testing kits gave a 30 percent false positive rate and a 20 percent false negative rate.