Labcorp test master test account 5450 millstream road mcleansville nc 27301 sample report 139900 patient details dob. 1 patient report specimen id. With its high mutation rate coronaviruses are zoonotic pathogens that are pres ent in humans and various animals with a wide range of.

With 80 160 nm in size and 27 32 kb positive polarity re combination rates of covs are very high because of con stantly developing transcription errors and rna depen dent rna polymerase rdrp jumps. Informasi terbaru seputar penanganan covid 19 di indonesia oleh pemerintah. An update to the guidance for the international.

Specific interim guidance on biosafety in the laboratory has also been published. Guidance is continually updated as more data becomes available and includes advice on sample collection diagnostic testing and pathogen characterization. Situation report 38 total and new cases in last 24.

The sample must arrive at the lab within 72 hours. Positive results are usually highly accurate but negative results may need to be confirmed with a molecular test. Air surface environmental and personal protective.

Ong sw tan yk chia py lee th ng ot wong ms et al. Some people can test positive for covid 19 from 1 3 days before they develop symptoms 6 16 thus it is possible that. Situation report 73 highlights.
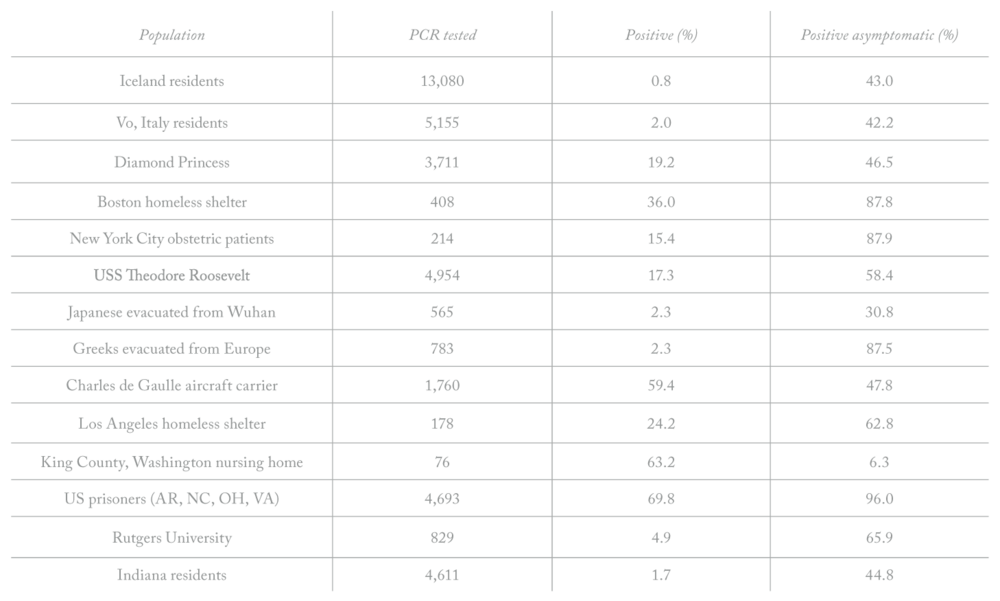
With our free coronavirus case report you can easily gather and organize these reports online so you can better protect your business or organization. A coronavirus case report allows people to report a suspected member of their workforce or community exhibiting covid 19 symptoms. The second report also shows a higher number of cases among men and patients aged above 50 years.

Of the 2 877 patients tested between march 22 and march 28 a total of 48 1 7 per cent were found positive.
Corona positive report sample pdf. The coronavirus aid relief and economic security cares act cares act section 18115 pdf icon external icon and its implementation guidance require every covid 19 testing site to report every diagnostic and screening test performed to detect sars cov 2 or to diagnose a possible case of covid 19 e g molecular antigen antibody to the appropriate state or local public health. To prevent further spread of sars cov 2 and to collect information to better understand the virus and its impact on health outcomes cdc is working with state and local health departments to identify persons under investigation pui in the united states and test them for the virus that causes covid 19. After the testing policy was changed to include sari patients only two of 106 patients were found positive between march 15 and march 21.

After the testing policy was changed to include sari patients only two of 106 patients were found positive between march 15 and march 21. To prevent further spread of sars cov 2 and to collect information to better understand the virus and its impact on health outcomes cdc is working with state and local health departments to identify persons under investigation pui in the united states and test them for the virus that causes covid 19. The coronavirus aid relief and economic security cares act cares act section 18115 pdf icon external icon and its implementation guidance require every covid 19 testing site to report every diagnostic and screening test performed to detect sars cov 2 or to diagnose a possible case of covid 19 e g molecular antigen antibody to the appropriate state or local public health.